What is Hemophilia B ?
Patients affected with Hemophilia B suffer uncontrolled bleeding from any part of body from inside or from any damaged or wounded body part, some times without any bruise or any cut bleeding occurs from internal organs or oral cavity.
Symptoms of Hemophilia B
Patients affected with Hemophilia B suffer bleeding in joints, and soft tissue, bleeding in stomach and frequent bleeding from nose, bleeding through gums, person get bleeding even with slight scratch on skin or any body part. Surgery is one point where there is risk of bleeding. Bloody urine blood in urine are the symptoms of Hemophilia B
Treatment of Hemophilia B, what is treatment available for Hemophilia B
Hemophilia B Patients are given injections or intravenous dosage of factor ix, which is only treatment available till yesterday before US FDA gave approval for a novel gene therapy drug. The factor ix intravenous dosage required is frequent just like patients of diabetes needs insulin, in same manner patients affected with Hemophilia B are required to give intravenous factor IX dosage to maintain the body level of factor IX so that they don’t suffer from bleeding.
What is responsible for Hemophilia B
Hemophilia Bis a disease condition occurring due to faulty gene which do not produce factor IX which is responsible for blood clotting process of formation of semisolid blood clot, in absence of factor ix, uncontrolled bleeding from damaged tissue occurs, since the clot formation do not occur in Hemophilia B patients, sometimes the bleeding is spontaneous, from inner organs, intestine or oral cavity, etc., it can occur at any site.
Hemophilia B is X chromosome linked disease, which is inherited mostly predominantly to males, some female too suffers from Hemophilia B, patients do not have a gene that produce factor IX.
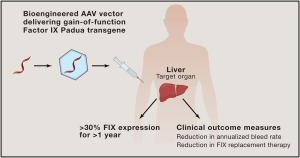
First of its kind New drug gene therapy approved by US FDA to treat Hemophilia B adult patients.
It’s a first of its kind of drug approved by US FDA, it is a Gene Therapy, name of the Gene therapy product is Hemgenix it is approved by US FDA on 22-11-2022 for treatment of adult Hemophilia B patients.
Hemgenix is given in a single dose IV infusion, this gene therapy product consists of a virus vector carrying a gene for factor IX , which is not virulent to human, but when given in intravenous dosage it is able to deliver the gene to targeted delivery site in patient’s liver where gene is expressed for production of Factor IX, blood level of Factor IX is increased and patients risk of bleeding is eliminated.

Clinical evaluation of safety and efficacy of Hemgenix is done by its innovator company CSL (ASX: CSL) https://www.csl.com/
Center for Disease Control US have published example of real people living with Hemophilia.
Leave a Reply