USFDA approves new drug that halts progression of Type 1 Diabetes.
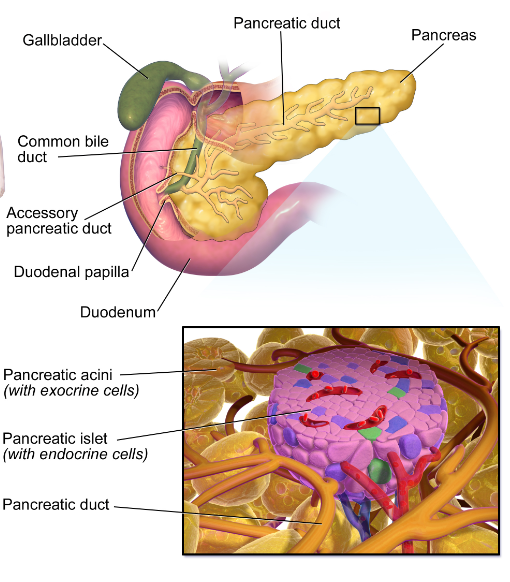
Type 1 diabetes is a very severe type of diabetes, type 1 diabetes patient’s pancreases produce very little of negligible amount of insulin which makes them diabetic, therefore patients affected with type 1 diabetes are required to take insulin injections throughout their lifetime.
One of the painful part is that it affects children and youngsters who are diagnosed at younger age for type 1 diabetes mellitus. Their pancreases beta cells of Langerhans which produce insulin are under attack from their own body’s defense system which is called autoimmune disease.
Patients pancreases is under attack by his own body’s antibodies which trigger a severe flare of secretions of interleukin 6 which in turn destroy the islets of langerhans beta cells.
The US FDA has approved the drug Tzield (teplizumab-mzwv) which can halt progression of type 1 diabetes from stage 2 to stage 3. Drug Tzield (teplizumab-mzwv) protects patient’s pancreatic cells from getting destroyed by autoimmune disorder. The autoimmune antibodies which are produced against the pancreatic beta cells of langerhans are neutralized with the drug Tzield (teplizumab-mzwv) which protects the cells producing insulin.
Drug Tzield (teplizumab-mzwv) is an injection containing monoclonal antibodies that is given through intravenous injection for 14 days to patients affected with type 1 diabetes.
Every drug is associated with its own side effects; the drug Tzield (teplizumab-mzwv) too has fewer cautions to be taken for mitigation of Cytokine Release Syndrome which some patients might get. Also certain white blood cell counts may decrease so it should be treated likewise.
Leave a Reply