Thalidomide the drug that caused limbless babies tragedy.
A Tale of Drug Approval, Tragedy, and the FDA Lady Who Saved Millions of American Children.
Thalidomide the drug that caused limbless babies, a drug initially hailed as a miracle medication, turned into one of the most notorious medical tragedies of the 20th century. Its journey from widespread approval in Europe to its rejection in the United States, followed by the emergence of a heroic FDA reviewer who prevented a potential catastrophe, is a gripping story that continues to resonate in the field of drug regulation and patient safety.

Thalidomide Approval in Europe:
In the late 1950s and early 1960s, thalidomide was marketed in Europe as a sedative and anti-nausea drug, gaining immense popularity due to its supposed efficacy and safety. Pregnant women, in particular, turned to thalidomide to alleviate morning sickness and insomnia, believing it was a harmless remedy. Its widespread use led to an unprecedented disaster that would affect thousands of families worldwide.
Thalidomide Tragedy:
The tragedy associated with thalidomide lay in its severe teratogenic effects. Teratogens are substances that can cause birth defects, and it was discovered too late that thalidomide could lead to devastating limb deformities and other developmental abnormalities in babies born to mothers who had taken the drug during pregnancy.
Thousands of infants were born with phocomelia, a condition characterized by severely shortened or absent limbs, leading to profound physical and emotional hardships. The tragedy sparked a global health crisis and sparked a new era in drug regulation, emphasizing the need for rigorous safety testing before approving medications for public use.
Thalidomide in the United States:
In the early 1960s, thalidomide’s manufacturer sought approval from the U.S. Food and Drug Administration (FDA) to market the drug in the United States. However, the FDA, led by one courageous woman, Dr. Frances Kelsey, took a cautious approach and declined to approve the drug.
The FDA Lady Who Saved Millions of American Children:
Dr. Frances Kelsey, a pharmacologist and medical officer at the FDA, was assigned the task of reviewing the application for thalidomide’s approval. Despite mounting pressure from the pharmaceutical company and her superiors to expedite the process, Dr. Kelsey remained steadfast in her commitment to patient safety and demanded further evidence regarding the drug’s safety profile.
As she scrutinized the available data, Dr. Kelsey became increasingly concerned about the lack of comprehensive research on thalidomide’s potential side effects. She was particularly cautious about the drug’s potential impact on pregnant women and their unborn children. Her persistence in seeking additional information and her refusal to rush the approval process ultimately prevented thalidomide from being licensed in the United States.
Months after Dr. Kelsey’s relentless efforts, reports of thalidomide’s devastating effects began to emerge from Europe, confirming her worst fears. It was clear that her unwavering dedication had saved countless American children from the same fate.
Legacy and Impact:
The thalidomide tragedy, thalidomide the drug that caused limbless babies a profound impact on drug regulation and prompted significant changes in medical and pharmaceutical practices worldwide. Stringent guidelines were implemented to ensure comprehensive safety testing of new drugs before approval, and the importance of vigilant pharmacovigilance and continuous monitoring of medications was emphasized.
Dr. Frances Kelsey’s unwavering commitment to patient safety and her pivotal role in preventing the thalidomide disaster earned her numerous accolades and recognition. In 1962, President John F. Kennedy awarded her the President’s Award for Distinguished Federal Civilian Service, and she became a role model for many women pursuing careers in science and medicine.
Current status of drug thalidomide:
Currently thalidomide is approved for treatment of certain cancers, it’s property or mode of action that stops formation of new blood vessels in a growing tissue kills the cancer cells. Hence it is used to treat cancer in hospitalised patients.
Molecular Structure of Thalidomide:
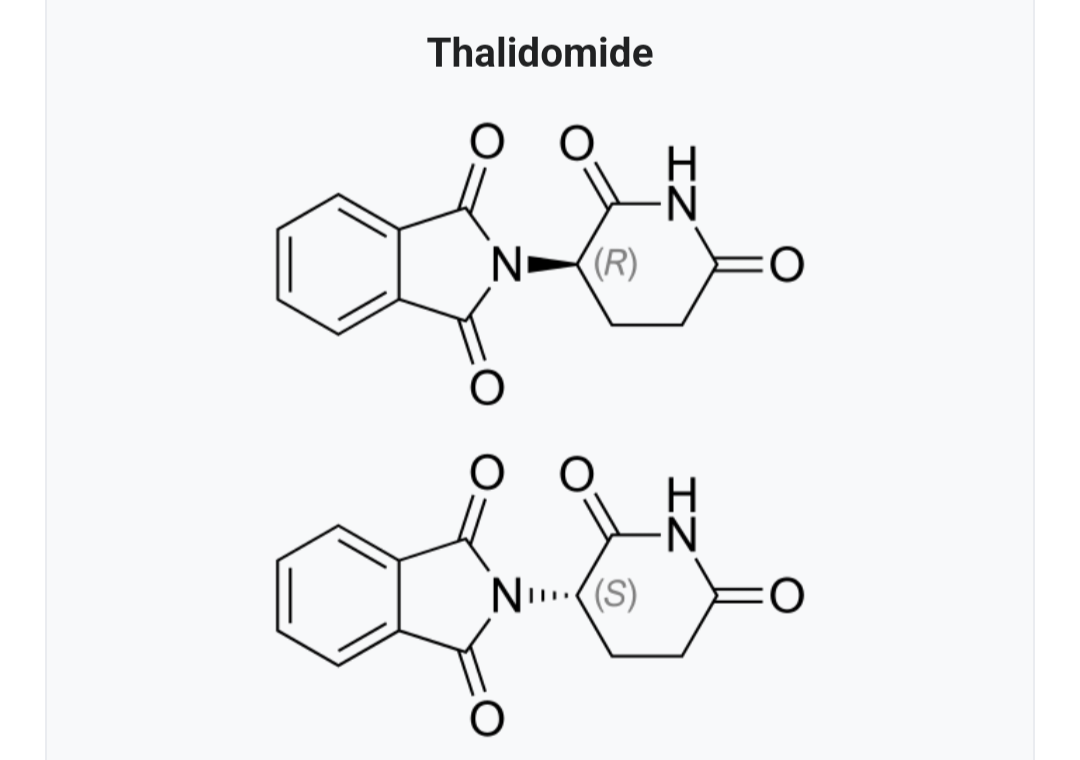
Thalidomide’s molecular formula is C13H10N2O4, and its chemical name is 2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione. Structurally, it is an organic compound consisting of two key moieties: an isoindole ring and a phthalimide ring. The molecule is a racemic mixture, which means it exists in two mirror-image forms (enantiomers) that exhibit different pharmacological activities.
Structural Activity Relationship (SAR) of Thalidomide:
Thalidomide’s SAR is crucial to understanding its therapeutic and teratogenic properties. The two enantiomers of thalidomide have distinct effects on the human body. One enantiomer is responsible for its sedative and antiemetic (anti-nausea) effects, while the other enantiomer is responsible for its teratogenicity.
It was later discovered that the S(-) enantiomer is primarily responsible for thalidomide’s teratogenic effects, leading to limb deformities in developing fetuses. Conversely, the R(+) enantiomer, while also possessing some teratogenicity, contributes to the drug’s therapeutic properties.
Mode of Action of thalidomide:
Thalidomide’s mode of action is multifaceted, and its exact mechanisms are not fully understood. However, its therapeutic effects have been linked to its ability to modulate the immune system and inhibit the production of certain cytokines, particularly tumor necrosis factor-alpha (TNF-α). TNF-α is a pro-inflammatory cytokine involved in various diseases, and thalidomide’s ability to reduce its levels has shown benefits in treating certain conditions.
In certain autoimmune and inflammatory disorders, thalidomide helps suppress excessive immune responses, leading to a reduction in symptoms. Additionally, it has anti-angiogenic properties, meaning it can inhibit the formation of new blood vessels, which can be beneficial in certain cancer treatments.
Current Uses of Thalidomide:
Despite its dark past, thalidomide has found its way back into medical practice due to its therapeutic potential in specific conditions. Some of its current uses include:
Multiple Myeloma:
Thalidomide, often in combination with other medications, is used in the treatment of multiple myeloma, a type of blood cancer affecting plasma cells.
Erythema Nodosum Leprosum (ENL):
Thalidomide is an FDA-approved treatment for ENL, a severe complication of leprosy, providing relief from skin lesions and inflammation.
Crohn’s Disease:
In some cases, thalidomide has been used to manage the symptoms of Crohn’s disease, a chronic inflammatory bowel disorder.
Behçet’s Disease:
Thalidomide has shown efficacy in managing certain manifestations of Behçet’s disease, a rare autoimmune disorder.
Contraindications:
While thalidomide has demonstrated therapeutic benefits, it remains a drug with potentially severe side effects, particularly when used during pregnancy. Therefore, it is essential to identify and adhere to contraindications to ensure patient safety.
Contraindications for thalidomide use include:
Pregnancy:
Thalidomide is strictly contraindicated in pregnant women or women of childbearing potential who are not using adequate contraception. Its teratogenic effects can lead to severe birth defects.
Hypersensitivity:
Individuals with a known hypersensitivity to thalidomide or any of its components should not use this medication.
Neuropathy:
Thalidomide can cause peripheral neuropathy, so caution should be exercised when prescribing it to patients with pre-existing nerve damage.
Active Infections:
Thalidomide’s immunomodulatory effects may interfere with the body’s ability to combat infections, making it potentially harmful in active infections.
History of Blood Clots:
Thalidomide may increase the risk of blood clots, and individuals with a history of thromboembolic events should avoid its use.
HIV Infection:
Thalidomide may exacerbate HIV infection, and its use is generally avoided in individuals with untreated or progressive HIV.
Conclusion:
The thalidomide tragedy serves as a powerful reminder of the critical role of drug regulators and the importance of thorough safety evaluation in pharmaceutical development. Dr. Frances Kelsey’s heroic actions at the FDA saved countless American children from a heartbreaking fate and revolutionized drug approval processes worldwide. Her legacy continues to inspire a commitment to patient safety and the pursuit of scientific excellence in the medical community, ensuring that such a tragedy is never repeated.

Leave a Reply