Clean Room Validation Microbial Count Limits method settle plate contact plate viable bacterial fungal count limits frequency of validation.
Classification of clean rooms in pharmaceutical manufacturing companies is part of validation activity, a clean room is designed to provide the desired level of clean grade environment. but it’s rated only to a particular grade after it is validated that the clean room is providing the desired level of particle count. As per the ISO 14644-2 and ISO 14644-3 specifications for validation of clean room.
European Union Guidelines for Pharmaceutical Clean Rooms have provided the limits for viable bacterial and fungal count. Which is provided below.
The European Union Guidelines also require that the limits are met at working as well as at rest conditions for the clean room.
Process of validation of a clean room should be planned in advance, an activity similar to the product manufacturing can be mimicked and particle count should be done at the points as per the guidelines provided in ISO 14644-2 and 3 specification.
Microbial Limits and Methods for Settle Plate and Contact Plates evaluation of Clean Room Viable Microbial Count for Validation of Clean Room.
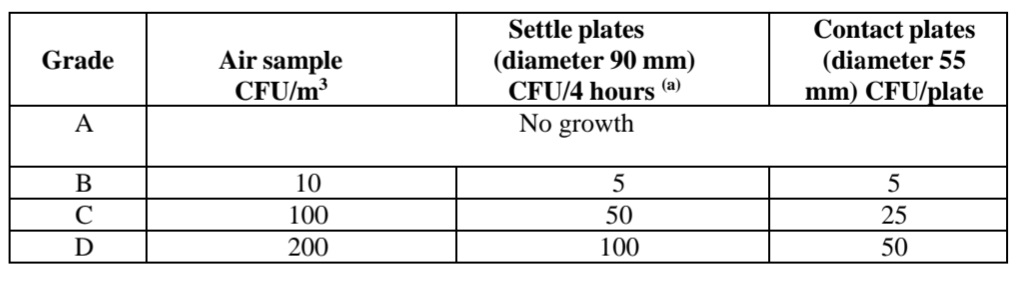
Microbial count is done by the settle plate method, plates are exposed for the duration of the process of manufacturing or not more than 4 hours, media plates should not get dried during exposure.
After cleaning the surfaces of the clean room and floor with detergent and water, followed by disinfectants.
Contact plate is used to take viable microbial counts from the surfaces of a clean room and growing and hand gloves of personnel working in the classroom. Both bacterial and fungal count is required to be taken, for bacterial and fungal count microbial nutrient media plates for contact plate method have different Colour for identification pink Colour is given for Fungal count plate.
Previously sterilized with Gamma radiation, prefilled with media, contact plates are provided with three outer bags of polythene. When these plates are taken into the Airlock of the Clean Room upper two bags are removed. Which ensures that outer contamination is removed. The inner bag is sterile and is removed just before the contact plate sample is taken from the surface. Place is placed over the surface with equal pressure and removed and the lid is covered.
Some contact plates come with locks on bottom and cap plate, when it is closed it provides anaerobic conditions inside the plate.
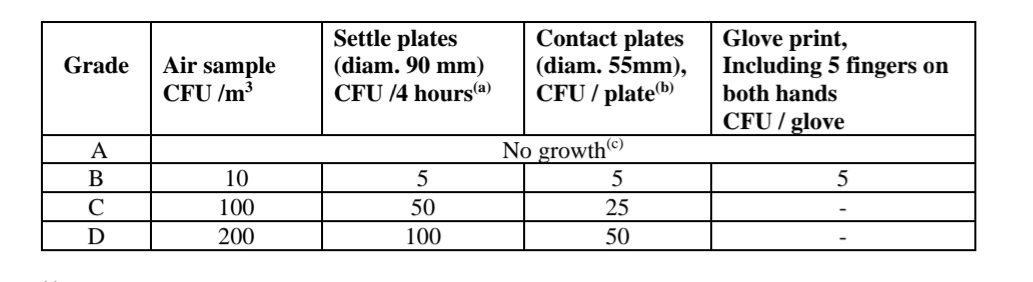
During qualification of clean room at working condition the microbial count from the personal gowning and hand is taken, for class A it should be zero. Any increase in microbial count should be investigated and root cause should be identified.
Clean Room Validation Microbial Count Action Limits for Surfaces.
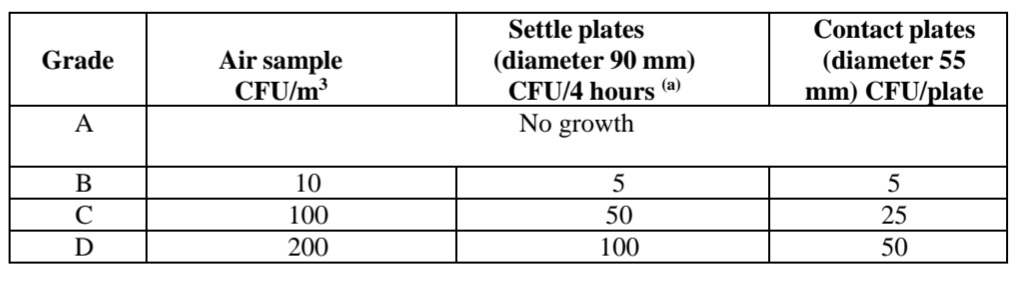
Clean Room Revalidation Frequency.
For Class A and B Clean Room Validation Frequency provided in guidelines is Six months while for Grade C and Grade D Clean Room it is 12 months.
Leave a Reply