Principles of Working of Selective Media in Microbiology.
Why microbial media are selective media and what is the principle behind their selectivity. Microbiolocal Selective media working principles. Selective media are essential tools in microbiology used to isolate and differentiate specific groups of microorganisms from mixed samples. These media contain various components that favor the growth of certain microorganisms while inhibiting others. Different selective media are employed for different purposes, depending on the type of microorganism being sought or the specific diagnostic goal. In this article, we will explore the principles of working of several selective media commonly used in microbiology, including MacConkey Agar, Mannitol Salt Agar, Blood Agar, EMB Agar (Eosin Methylene Blue Agar), CNA Agar (Columbia Colistin Nalidixic Acid Agar), XLD Agar (Xylose Lysine Deoxycholate Agar), and MRS Agar (De Man, Rogosa, and Sharpe Agar).
MacConkey Agar:
MacConkey Agar is a selective and differential medium used for the isolation and differentiation of gram-negative bacteria, especially members of the Enterobacteriaceae family. It contains bile salts and crystal violet, which inhibit the growth of gram-positive bacteria. Additionally, the medium contains lactose and neutral red indicator. Lactose-fermenting bacteria produce acid during fermentation, leading to the formation of pink to red colonies, whereas non-lactose fermenters produce colorless or pale colonies.
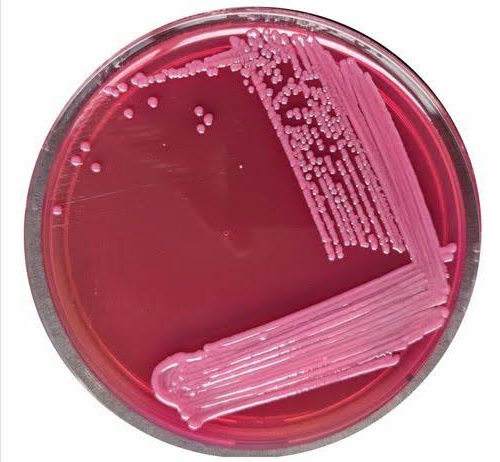
MacConkey Agar principle of selectivity:
MacConkey Agar inhibits the growth of other gram-positive microorganisms due to the presence of selective components in the medium. The key inhibitory agents in MacConkey Agar are bile salts and crystal violet. These components exert their inhibitory effects in the following ways:
Bile Salts: Bile salts are natural substances produced by the liver and stored in the gallbladder. In MacConkey Agar, the addition of bile salts creates an inhibitory environment for most gram-positive bacteria.Bile Salt is a surface active agent it lowers the surface tension between the bacterial cell wall hence the gram positive bacterias don’t survive in low surface tension environment. Gram-positive bacteria lack an outer membrane, which makes them more susceptible to the disruptive action of bile salts. Bile salts can destabilize the cell membrane, disrupt cell integrity, and lead to cell lysis in gram-positive bacteria.
Crystal Violet: Crystal violet is a synthetic dye that is added to MacConkey Agar to further inhibit the growth of gram-positive bacteria. It is especially effective against those gram-positive bacteria that are not as sensitive to bile salts alone. Crystal violet interacts with the peptidoglycan layer of the cell wall in gram-positive bacteria, causing structural damage and interfering with cellular functions.
As a result of these selective agents, MacConkey Agar specifically supports the growth of gram-negative bacteria, particularly members of the Enterobacteriaceae family, while suppressing the growth of most gram-positive bacteria.
Mannitol Salt Agar:
Mannitol Salt Agar is a selective medium used for the isolation and identification of staphylococci, particularly Staphylococcus aureus. It contains a high concentration of salt (7.5%-10%) that inhibits the growth of most other bacteria. The medium also contains mannitol and phenol red indicator. Staphylococcus aureus ferments mannitol, producing acid and causing the medium to change from red to yellow.
Mannitol Salt Agar principle of selectivity:
Mannitol Salt Agar inhibits the growth of most bacteria, including gram-negative microorganisms, due to its high salt concentration (7.5%-10%). The high salt concentration creates a hypertonic environment that is selective against a wide range of microorganisms. The mechanisms behind its selective nature are as follows:
High Salt Concentration: The elevated salt concentration in Mannitol Salt Agar is particularly inhibitory to many bacteria, including gram-negative species. Most bacteria find it difficult to grow in such a high-salt environment due to the osmotic stress it imposes on their cells.
Despite the inhibitory effect of the high salt content, Mannitol Salt Agar is specifically formulated to support the growth of staphylococci, especially Staphylococcus aureus. Staphylococcus aureus is one of the few microorganisms that can tolerate the high salt concentration and grow under these conditions. The medium also contains mannitol as a fermentable carbohydrate and phenol red as a pH indicator, allowing for the differentiation of Staphylococcus aureus based on its ability to ferment mannitol and produce acid.
Blood Agar:
Blood Agar is a general-purpose medium enriched with sheep or horse blood. It supports the growth of a wide range of bacteria and fungi and is used for various purposes, including determining hemolytic activity. Hemolysis, the breakdown of red blood cells, can be observed as different types on this medium: alpha-hemolysis (partial hemolysis, greenish zone), beta-hemolysis (complete hemolysis, clear zone), and gamma-hemolysis (no hemolysis, no change in the medium).
Blood Agar is not selective against specific groups of microorganisms and does not contain any inhibitory agents to prevent the growth of certain bacteria. Instead, it is an enriched medium due to the addition of blood, which provides essential nutrients and growth factors for a wide variety of bacteria and fungi. The blood in Blood Agar enhances the growth of fastidious organisms, which are microorganisms that have specific nutritional requirements for growth and are often difficult to cultivate on standard media.
The primary purpose of Blood Agar is to determine the hemolytic activity of bacteria. Hemolysis refers to the breakdown of red blood cells, and different types of hemolysis produce characteristic patterns on the medium: alpha-hemolysis (partial hemolysis, greenish zone around colonies), beta-hemolysis (complete hemolysis, clear zone around colonies), and gamma-hemolysis (no hemolysis, no change in the medium).
EMB Agar (Eosin Methylene Blue Agar):
EMB Agar is a selective and differential medium primarily used for the isolation and differentiation of gram-negative enteric bacteria. It contains lactose, eosin, and methylene blue. Lactose fermenters produce colonies with a dark center and a metallic green sheen due to acid production. Non-lactose fermenters appear colorless.
EMB Agar (Eosin Methylene Blue Agar) principle of selectivity:
EMB Agar is selective against gram-positive bacteria due to the presence of eosin and methylene blue, which inhibit their growth. The selective and differential properties of EMB Agar are as follows:
Eosin and Methylene Blue: Eosin and methylene blue are acidic dyes that inhibit the growth of most gram-positive bacteria. These dyes act on the cytoplasmic membrane of gram-positive cells, causing cell leakage and disruption.
Lactose and Neutral Red: EMB Agar contains lactose as a fermentable carbohydrate and neutral red as a pH indicator. Lactose-fermenting bacteria produce acid during fermentation, leading to the formation of dark-centered colonies with a metallic green sheen. Non-lactose fermenters appear colorless on the medium.
EMB Agar is primarily used for the isolation and differentiation of gram-negative enteric bacteria, such as Escherichia coli and Enterobacter species.
CNA Agar (Columbia Colistin Nalidixic Acid Agar):
CNA Agar is a selective medium used to isolate gram-positive cocci, particularly Streptococcus and Staphylococcus species. It contains colistin and nalidixic acid, which inhibit the growth of gram-negative bacteria. Additionally, it is supplemented with sheep blood for improved growth of fastidious organisms.
CNA Agar (Columbia Colistin Nalidixic Acid Agar) principle of selectivity:
CNA Agar is selective against most gram-negative bacteria due to the presence of the antibiotics colistin and nalidixic acid. The selective and differential properties of CNA Agar are as follows:
Colistin and Nalidixic Acid: Colistin and nalidixic acid are antibiotics that inhibit the growth of gram-negative bacteria. These antibiotics interfere with the integrity of the outer membrane of gram-negative bacteria, leading to cell death.
Sheep Blood: CNA Agar is enriched with sheep blood to support the growth of gram-positive cocci, particularly Streptococcus and Staphylococcus species.
The combination of selective agents in CNA Agar allows for the isolation and cultivation of gram-positive bacteria, while suppressing the growth of most gram-negative bacteria.
XLD Agar (Xylose Lysine Deoxycholate Agar):
XLD Agar is a selective and differential medium used for the isolation and differentiation of enteric pathogens, especially Salmonella and Shigella species. It contains xylose, lactose, sucrose, lysine, thiosulfate, and phenol red indicator. Salmonella produces black colonies due to hydrogen sulfide production and can ferment xylose, while Shigella does not ferment xylose and appears red.
XLD Agar (Xylose Lysine Deoxycholate Agar) principle of selectivity:
XLD Agar is selective against most gram-positive bacteria due to its selective components. The selective and differential properties of XLD Agar are as follows:
Xylose, Lactose, and Sucrose: XLD Agar contains xylose, lactose, and sucrose as fermentable carbohydrates. The ability of microorganisms to ferment these sugars leads to the formation of acid, which lowers the pH of the medium.
Lysine and Thiosulfate: XLD Agar contains lysine and thiosulfate as substrates for detecting hydrogen sulfide (H2S) production. Some microorganisms, like Salmonella, can produce H2S during metabolism.
Phenol Red: Phenol red is a pH indicator that changes color in response to changes in pH. Acid production causes the medium to turn yellow, while alkaline reactions result in red or pink colors.
XLD Agar is primarily used for the isolation and differentiation of enteric pathogens, especially Salmonella and Shigella species.
MRS Agar (De Man, Rogosa, and Sharpe Agar):
MRS Agar is a selective medium used for the isolation and enumeration of Lactic Acid Bacteria (LAB), especially Lactobacillus species. It contains peptone, yeast extract, dextrose, polysorbate 80, and a phosphate buffer. The medium is slightly acidic, promoting the growth of LAB while inhibiting the growth of most other bacteria.
MRS Agar (De Man, Rogosa, and Sharpe Agar) principle of selectivity:
MRS Agar is selective for Lactic Acid Bacteria (LAB), particularly Lactobacillus species. The selective and differential properties of MRS Agar are as follows:
Polysorbate 80: MRS Agar contains polysorbate a surfactant that inhibits the growth of gram-negative bacteria. Polysorbate 80 disrupts the outer membrane of gram-negative bacteria, leading to cell lysis.
Slightly Acidic pH: MRS Agar is slightly acidic, promoting the growth of LAB, which thrive in such conditions. The acidic pH inhibits the growth of most other bacteria.
MRS Agar is commonly used for the isolation and enumeration of LAB in various food and fermentation processes.
Sabouraud Agar:
Composition:
Peptone: 1%
Dextrose (Glucose): 4%
Agar: 2%
Distilled water
Sabouraud Agar is a selective medium used for the isolation and cultivation of fungi. It was developed by Raymond Sabouraud in the late 19th century and has since become one of the most commonly used media for fungal studies.
Principle of Selectivity Sabouraud Agar:
The selective nature of Sabouraud Agar is primarily due to its low pH, which inhibits the growth of many bacteria, making it more favorable for fungal growth. The acidic pH of the medium (around 5.6) is achieved by using dextrose as the carbohydrate source. Most bacteria are unable to thrive in such an acidic environment, providing a selective advantage for fungi.
Additionally, Sabouraud Agar may contain antibiotics like chloramphenicol or gentamicin to further inhibit bacterial growth and ensure the dominance of fungal colonies.
Lowenstein-Jensen Agar:
Composition:
Asparagine: 0.5%
Glycerol: 0.8%
Malachite Green: 0.025%
Agar: 2%
Distilled water
Lowenstein-Jensen Agar is a selective medium used for the isolation and cultivation of Mycobacterium species, particularly Mycobacterium tuberculosis, the causative agent of tuberculosis.
Lowenstein-Jensen Agar Principle of Selectivity:
The selective nature of Lowenstein-Jensen Agar is primarily attributed to the presence of malachite green, which inhibits the growth of most bacteria and fungi, making it ideal for the isolation of slow-growing Mycobacterium species.
Mycobacteria are characterized by their slow growth rate, and they require special nutrients and conditions for their cultivation. The presence of asparagine and glycerol in Lowenstein-Jensen Agar provides essential nutrients that support the growth of Mycobacterium species, while the malachite green inhibits the growth of most other microorganisms.
Hektoen Enteric Agar:
Composition:
Lactose: 1%
Sucrose: 1%
Salicin: 0.2%
Sodium Thiosulfate: 0.08%
Ferric Ammonium Citrate: 0.2%
Bromothymol Blue: 0.065%
Acid Fuchsin: 0.3%
Agar: 0.75%
Distilled water
Hektoen Enteric Agar is a selective and differential medium used for the isolation and differentiation of Salmonella and Shigella species from clinical samples and food samples.
Hektoen Enteric Agar Principle of Selectivity:
The selective nature of Hektoen Enteric Agar is achieved through the incorporation of bile salts, which inhibit the growth of most gram-positive bacteria, including some non-enteric gram-negative bacteria. This inhibitory effect creates a selective environment for the growth of enteric pathogens like Salmonella and Shigella.
Hektoen Enteric Agar Principle of Differential Properties:
Hektoen Enteric Agar contains multiple carbohydrate sources (lactose, sucrose, and salicin), which serve as substrates for fermentation by the target bacteria. Additionally, sodium thiosulfate and ferric ammonium citrate are included to detect hydrogen sulfide (H2S) production by some species. The pH indicator, bromothymol blue, changes color in response to acid production during fermentation.
Salmonella and Shigella species can ferment some of the carbohydrates, leading to the formation of acid. This results in the appearance of colonies with varying colors, such as yellow or green, based on their fermentation capabilities. H2S production is detected by the appearance of black colonies or black centers within the colonies.
Selective media working principles, Selective media are crucial tools in microbiology that allow the isolation and identification of specific groups of microorganisms from complex samples. Each selective medium’s unique composition provides specific conditions that inhibit the growth of unwanted microorganisms while promoting the growth of the target microorganisms, making them invaluable tools in diagnostic and research laboratories.
Leave a Reply