Pharmaceutical Area classification with respect to filters used as per WHO guidelines
WHO gmp guidelines describes area in a pharmaceutical Manufacturing facility, as Level 1 level 2 and level 3 areas.
Level 1 Area is place where there is no possibility of contamination of product, the product comes here after final packing if at all, this area is a general area which may be used for housekeeping and maintainace. (Non classified area)
Level 2 Area is area where the raw and packing material is stored in closed condition, this area is controlled with respect to temperature and humidity. These areas are not provided with coarse filters, the working benches and dispensing places may be provided with UDF units. (UDF unit is a unit having vertical laminar air flow) (Classified area)
Level 3. Is area where actually pharmaceutical ingredients raw material or under process product is exposed to environment, therefore this area is required to control for its quality of air and cleanness. This area is called as controlled area; these areas are supplied air filtered through HEPA filters. (Classified area)
Which Filters can be used as per WHO GMP guidelines for Level 1 level 2 and level 3 areas
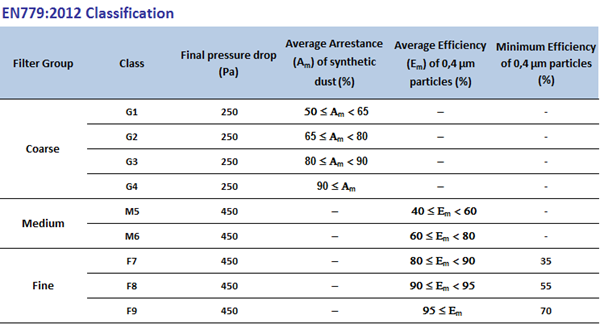
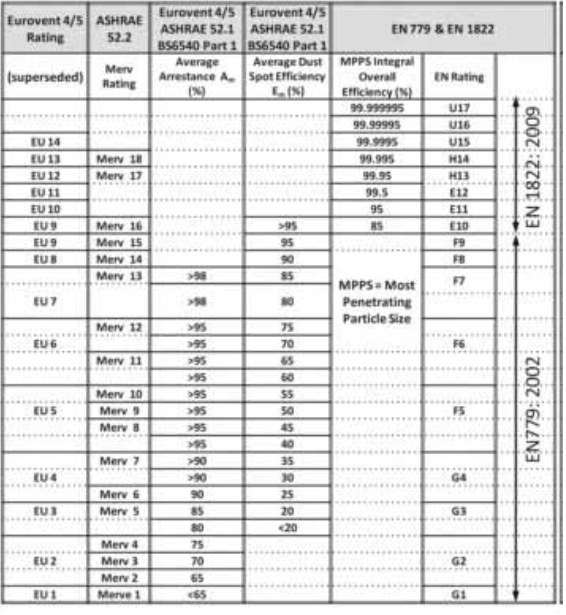
Level 1: EN 779 G4 filters Primary filters only
Level 2: EN 779 G4 plus F8 or F9 filters air used in this area is 100 % outside air being filtered and supplied.
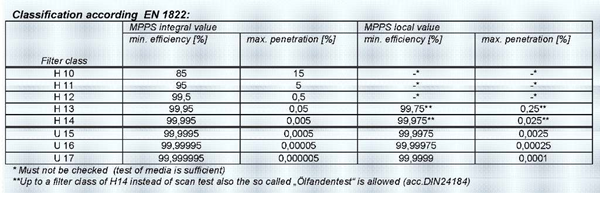
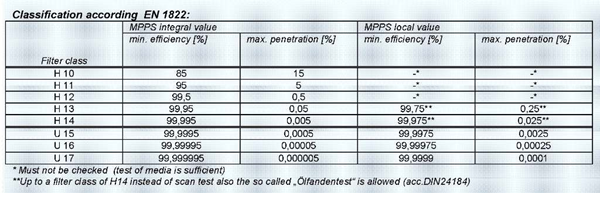
Level 3: EN 779 G4 plus F8 plus EN 1822 H13 filters. Recirculated air is used, where there is possibility of cross contamination hence three level filters are used as primary, secondary and tertiary filters.
Leave a Reply