New drug Leqembi containing lecanemab-irmb for treatment of Alzheimer’s disease approved by US FDA under accelerated approval pathway.
There are very few treatments available for treatment of Alzheimer’s disease, people affected with Alzheimer’s disease are not able to do daily tasks, and there is a risk of forgetting the way back to home when they go out of home.
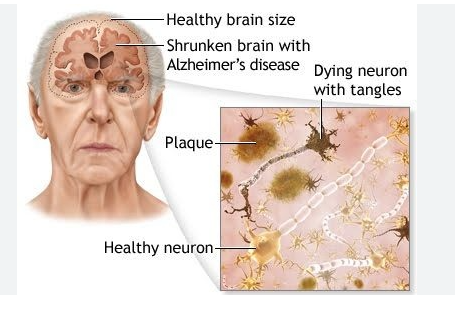
Pathophysiology of Alzheimer’s Disease
Alzheimer’s disease occurs due to loss of neurons in the brain and their connections to other neurons, as a result of increased amyloid beta plaques and neurofibrillary, or tau, tangles. Person affected with Alzheimer’s disease loses capability of thinking and memorizing things, and the patient loses memory. Disease is devastating to patients as well as family members of patients as one has to take care of the patient all the time.
The US FDA has approved Drug Leqembi containing lecanemab-irmb monoclonal antibodies against Amyloid beta, for treatment of early stages of Alzheimer’s disease. Drug Leqembi is not approved for late stages of disease. Leqembi is manufactured by Eisai R&D Management Co, it contains lecanemab-irmb is a monoclonal antibody consisting of immunoglobulin (IG g) which neutralizes gathered soluble and insoluble forms of amyloid beta plaques.
In a clinical trial safety and efficacy of Drug Leqembi was evaluated by giving patients affected with mild dementia with amyloid beta dosage of drug Leqembi (lecanemab-irmb) was given 10 mg / KG of body weight. every 14 days. It was observed that these patients who were receiving drug Leqembi (lecanemab-irmb) were found to be with reduction in brain amyloid beta plaque.
The drug Leqembi (lecanemab-irmb) acts on the basic root cause of Alzheimer’s disease, the brain amyloid plaque, therefore US FDA have approved this drug under accelerated approval pathway.
Side effects of the drug are headache, amyloid-related imaging abnormalities (ARIA), temporary swelling over the brain with mild bleeding, swelling ceases up on treatment, reactions associated with sterile dosage form and monoclonal antibody infusions. Drug Leqembi (lecanemab-irmb) is not approved for late stage Alzheimer’s disease.
Also see Sildenafil in treatment of dementia.
What is Alzheimer’s Disease
Leave a Reply