Month: March 2023
-
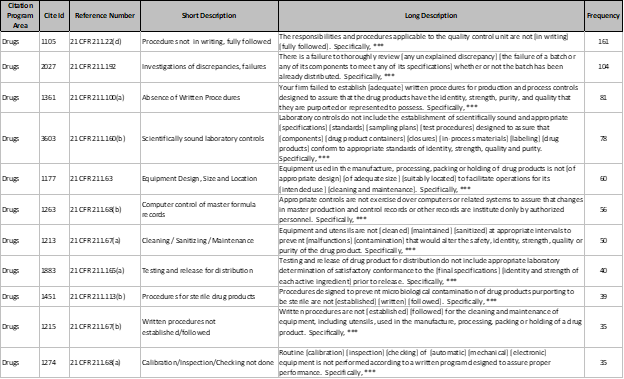
What is 483 observation of USFDA
What is 483 USFDA observations? Detail information issued by the US FDAs 483 observation form. US FDA is world one of best pharmaceutical regulatory agency, when it conducts any inspection and observe any discrepancy or violation with respect to the good manufacturing practices guidelines issued by US FDA, it provides the observations in a format…
-
How to Investigate Out of Speciation results
What is OOS in pharma, how to Investigate Out of Speciation results. OOS is an abbreviation of term “out of specification” the specification means the standards of the quality attributes like assay, measure of content in the formulation, Colour standard, written visual description, identification, and purity etc. When a product or a material falls out…
-

Compressed air gas for pharmaceutical use
Compressed air gas for pharmaceutical manufacturing it’s limit for microbial count. for sterile product the Total Viable Bacterial count should be lesser or equal to 1 cfu/ cubic metre, and Total fungal count should be nil or not detected, the particle count of compressed air should equal to the at rest condition of the respective…
-
Hold time study in pharmaceutical manufacturing
Hold time study in pharmaceutical manufacturing. What is hold time study? Good manufacturing guidelines for pharmaceutical manufacturing mentions that a pharmaceutical manufacturing unit must follow time limitations for stages of manufacturing. US FDA guidelines have not provided a particular time as standard for hold time. Definition of hold time: It is a time period for…
-
Hold time study in cleaning validation
Hold time study for clean and unclean equipments must be carried out during cleaning validation. It’s one of important aspect that many pharma companies forget to do Hold time study for clean and unclean equipments during Cleaning Validation. What is Clean equipment hold time dirty equipment hold time ? During validation of cleaning procedures, hold…
-

Cleaning Validation In Pharma
Cleaning validation in pharma manufacturing. When you thinking of doing cleaning validation of an equipment or a procedure adapted for cleaning, first question you should ask is that is the cleaning validation planed in the validation master plan? If not it’s not late to do that. Along with cleaning validation most important work needs to…
-
New Tuberculosis Vaccine developed that does not require to store at cool storage
New Tuberculosis Vaccine which is a subunit vaccine for TB developed that does not require to store at cool cold storage found the problems with live attenuated TB vaccine can be eliminated with this new vaccine. Tuberculosis vaccine consist of live bacteria, mycobacterium bovis, its virulence is reduced by the process of attenuation, vaccine is…
-
Recall Classification Mock Recall
Recall Classification Mock Recall detail information. Q: Why are Pharmaceutical Product is Recalled by pharmaceutical manufacturing companies? Q: What is the Mock recall? How mush is Time limit set by FDA for recall? Classification of Recall in pharmaceutical. Give procedure for doing mock recall. Q: What is type1, 2,3 recall or What is Class A,B,C…